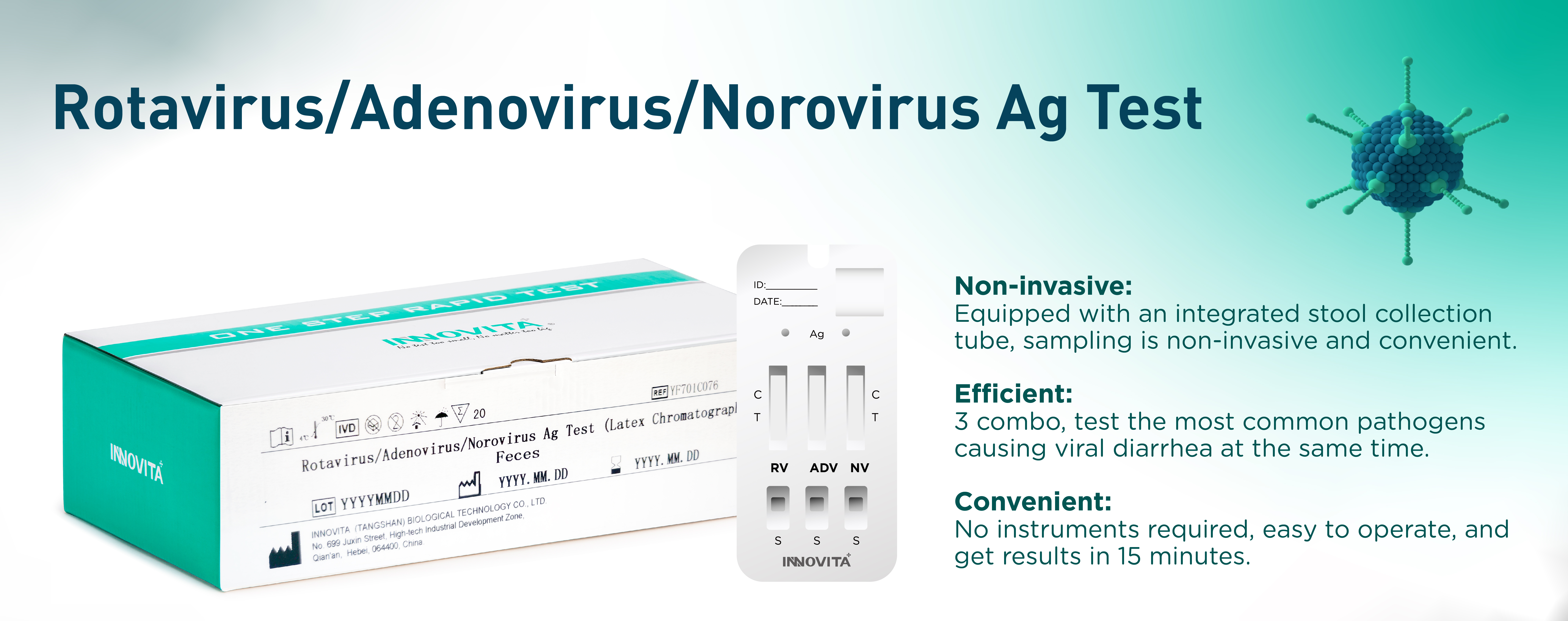
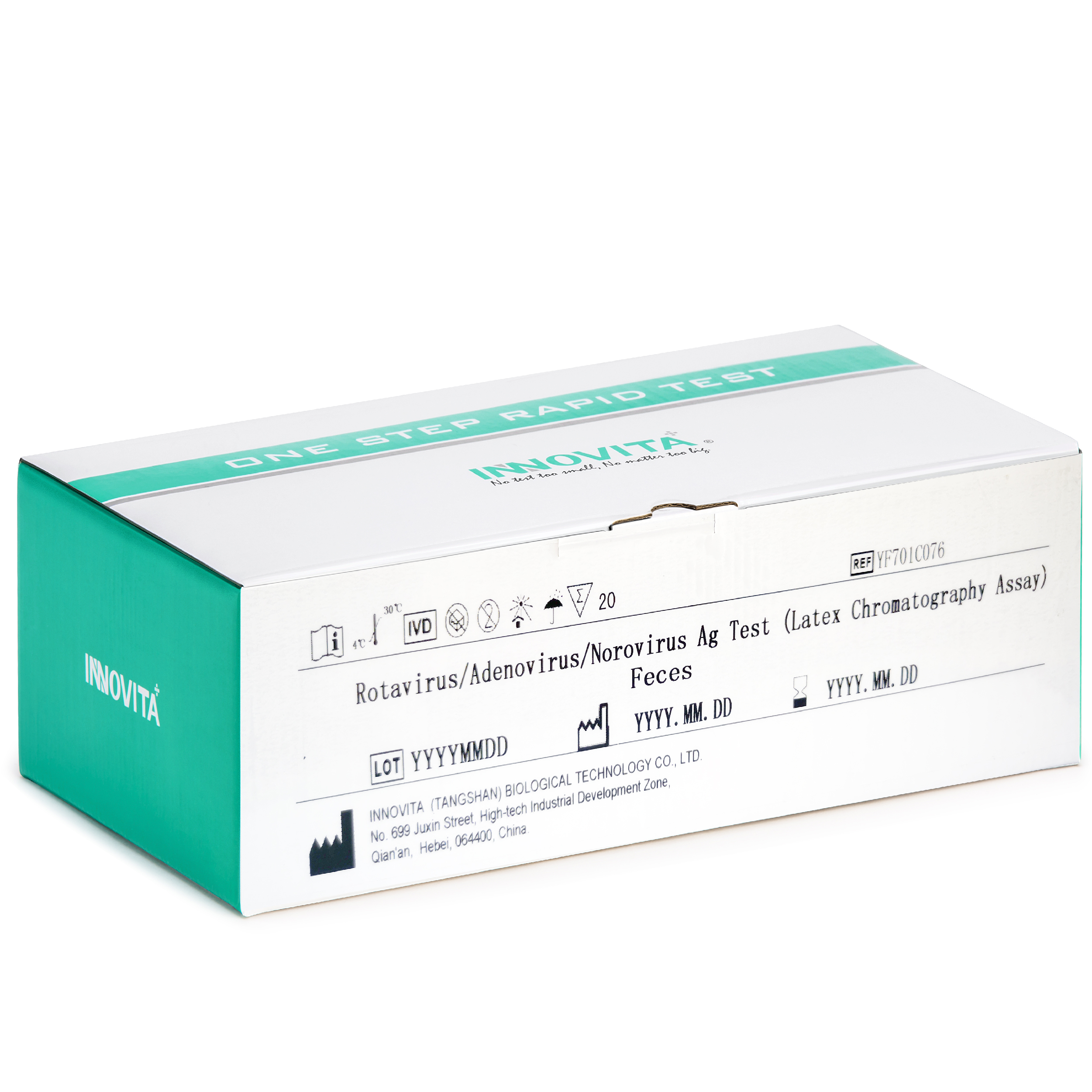
Innovita Biological Technology Co., Ltd. (together with its subsidiaries, collectively known as “INNOVITA”,) is a biotechnology company focusing on the research, development, production and sales of in vitro diagnostic POCT products. It consists of Innovita (Beijing) , Innovita (Tangshan) and Innovita (Guangzhou).
● Founded in 2006
● On 28 July 2022 INNOVITA completed initial public offering and shares began trading on SSE STAR market
Our Products
we advice to choose
Innovita
- Our Power
- OUR R&D Strength
- Our Quality Certification
INNOVITA is a leading manufacturer of diagnostic solutions for healthcare, striving for a more efficient healthcare system to enhance the health and well-being of people all over the world.
INNOVITA has built six technical platforms such as antigen & antibody preparation, virus culture, colloidal gold, ELISA, fluorescence chromatography, immunofluorescence, and undertakes Chinese National High-tech R&D Program in the field of national major infectious diseases research and many other public health projects.
INNOVITA adheres to GMP Standards and owns 100,000 class clean rooms to ensure the safety and hygiene of our products.
We strictly implement ISO 13485 quality management system and follow the standards and regulations of EU, FDA, etc., to ensure that every product meets the requirements of our customers worldwide.

we’ll ensure you always get
best results
-

SET UP 2006 -

EMPLOYEES 400+ -

COUNTRIES/REGIONS 70+ -
BRANCHES 3
Product Categories
Inquiry for pricelist
submit nowlatest news
view more-

1st exhibition at the beginning of 20...
The 4-day 2023 Dubai MEDLAB was successfully concluded at the Dubai World Trade Center on February 9. 747 exhibitors from 48 countries around the world gathered here to showcase the latest products... -

Upcoming Event: MEDLAB Middle East
MEDLAB Middle East has scheduled a new edition for 2023. MEDLAB Middle East 2023 will bring together experts and trade visitors for an in-person appointment from 6 to 9 February at Dubai World Tra... -

Upcoming Event: Medlab Asia & As...
Medlab Asia, along with the accompanying Asia Health event, will return in 2022. Medlab Asia & Asia Health 2022 will occur from 19 to 21 October 2022 and will be co-organised by Impact Muang T...